Triaminic® Syrups and Theraflu Warming Relief® Syrups
Description: This recall involves Triaminic® Syrups and Theraflu Warming Relief® Syrups for coughs, colds and fevers. There are 24 types of these two products included in the recall. A complete list of products, lot numbers and National Drug Codes (NDC) can be found at www.novartisOTC.com. Lot numbers are located on the bottom panel of the box and on the left side of the label on the bottle. The NDC number is located on the upper right corner of the front panel of the Triaminic Syrups box and the upper left corner of the Theraflu Warming Relief Syrups bottle. [Learn More]
About 2.3 Million units were affected by this recall.
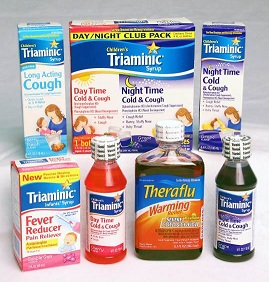
Triaminic and Theraflu Products Recalled Due to Failure to Meet Child-Resistant Closure Requirement; Risk of Poisoning
Triaminic Syrups and Theraflu Warming Liquids.
Triaminic® Syrups and Theraflu Warming Relief® Syrups Recall Information
| Country of Origin | United States |
| Recall Date | 01/31/2013 |
| Recall ID | 1310 |
| Recall Number | 13114 |
| Product Type | Other Drugs or Medications |
| Hazard | These child-resistant caps can fail to function properly and enable the cap to be removed by a child with the tamper-evident seal in place, posing a risk of unintentional ingestion and poisoning. These products contain acetaminophen and diphenhydramine which are required by the Poison Prevention Packaging Act to be sealed with child-resistant packaging. |
| Injuries | The firm has received 12 reports of children unscrewing the locked caps, including four reports of children ingesting the product. One child required medical attention. |
| Remedy | Consumers should immediately stop using the recalled product and contact Novartis for instructions on how to return the product for a full refund. |
| Remedy Option | Refund |
| Contact | Novartis Consumer Healthcare toll-free at (866) 553-6742 from 8 a.m. to midnight ET, Monday through Saturday, or online at www.novartisOTC.com for more information. |
| Last Updated | 01/20/2015 |
| Similar To |
