Zolpidem Tartrate Sublingual tablets
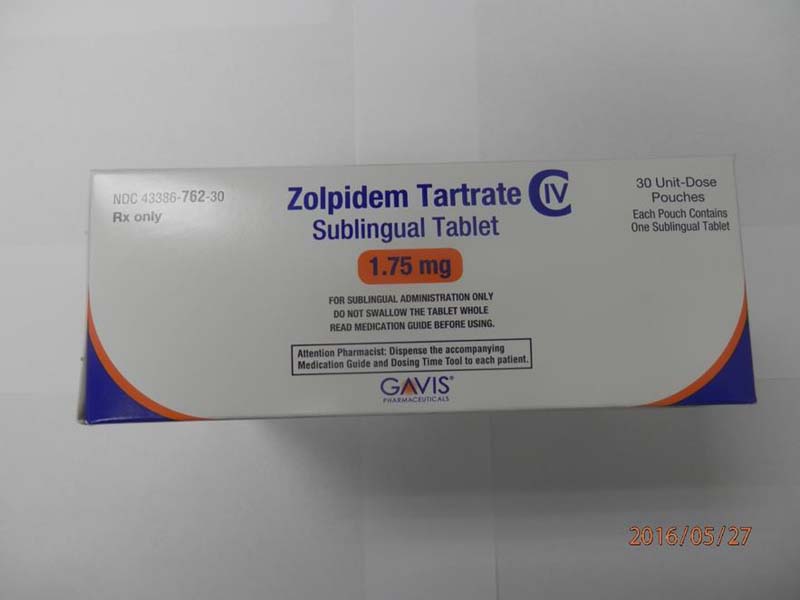
Description: This recall involves 1.75 mg and 3.5 mg Zolpidem Tartrate sublingual (rapid dissolve) tablets from Novel Laboratories. The prescription medication is a sleep drug. The packaging for the 1.75 mg tablets has lot number M16140A, 1.75 mg and national drug code (NDC) 43386-762-30. The packaging for the 3.5 mg tablets has lot number M16144A, 3.5mg and national drug code (NDC) 43386-761-30. The Zolpidem Tartrate is packaged as a single tablet in a peel-and-push blister pack inside an outer open-ended pouch with 30 pouches per box. The lot number and an expiration date of 02/2018 are printed on the bottom left of the pouches. The NDC is printed on the top left corner of the boxes. "Gavis" is printed in blue on the center of the white boxes and pouches. [Learn More]
About 5,700 boxes of 30 blister packs units were affected by this recall.
Novel Laboratories Recalls Zolpidem Tartrate Blister Packs Due to Failure to Meet Child-Resistant Closure Requirement; Risk of Poisoning
Novel Laboratories sleep tablets 1.75 mg box (front). Novel Laboratories sleep tablets 1.75 mg envelope (front)
Zolpidem Tartrate Sublingual tablets Recall Information
| Country of Origin | United States |
| Recall Date | 07/27/2016 |
| Recall ID | 7814 |
| Recall Number | 16232 |
| Product Type | Tablet or Capsule Drugs |
| Hazard | The packaging for the prescription drug is not child-resistant as required by the Poison Prevention Packaging Act, posing a risk of poisoning if swallowed by children. |
| Injuries | None reported |
| Remedy | Consumers should immediately stop using the recalled tablets and contact Novel Laboratories for instructions to receive a full refund. |
| Remedy Option | Refund |
| Contact | Novel Laboratories toll-free at (866) 403-7592 from 9 a.m. to 5 p.m. ET Monday through Friday or online at www.novellabs.net and click on “Important Zolpidem Information” for more information. |
| Last Updated | 07/27/2016 |
| Similar To |
|
