Cassin Blade Runner and Blade Runner Alpine Crampons
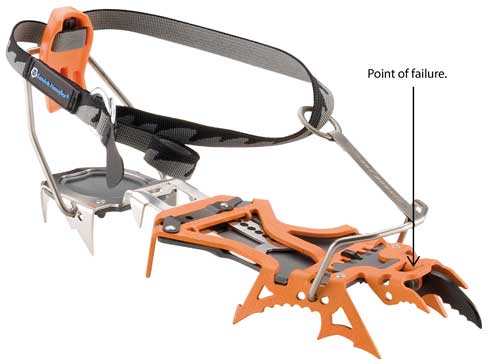
Description: This recall involves all Cassin Blade Runner and Blade Runner Alpine crampons. Crampons are used for ice climbing and attach to footwear allowing the front points of the crampon to cut into the snow or ice. The Blade Runner crampons have two ice front points and the Blade Runner Alpine crampons have two snow front points. "Sandvik Nanoflex" is printed on a rubber label attached to the gray nylon ankle strap. "Cassin" and "Blade Runner" are stamped on the orange traction plate on the foot of the crampon. The crampon frames are made of Chromoly and Nanoflex® steel. [Learn More]
About 900 (in addition, 20 were sold in Canada) units were affected by this recall.
CAMP USA Recalls Blade Runner Crampons Due to Fall Hazard
Cassin Blade Runner Crampon. Cassin Blade Runner Alpine Crampon
Cassin Blade Runner and Blade Runner Alpine Crampons Recall Information
| Country of Origin | Italy |
| Recall Date | 08/26/2015 |
| Recall ID | 6490 |
| Recall Number | 15225 |
| Product Type | Mountain Climbing |
| Hazard | The front part, where the points connect to the crampons, can break, posing a fall hazard. |
| Injuries | CAMP USA has received three reports of these crampons breaking during use. No injuries have been reported. |
| Remedy | Consumers should immediately stop using these recalled crampons and contact the firm for instructions on receiving free replacement parts. |
| Remedy Option | Repair |
| Contact | CAMP USA toll-free at 877-421-2267 from 9 a.m. to 5 p.m. MT Monday through Friday or online at www.camp-usa.com and click on the Safety Notices link at the bottom of the page then click on Blade Runner Crampon for more information. |
| Last Updated | 08/26/2015 |
| Similar To |
