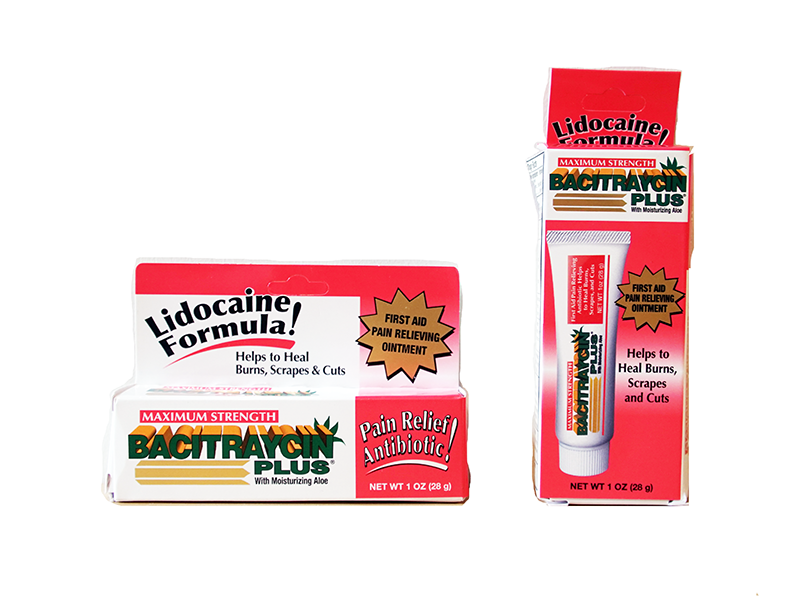
Maximum Strength Bacitraycin Plus Ointment with Lidocaine
Description: This recall involves Maximum Strength Bacitraycin Plus Ointment with Lidocaine. The recalled ointment is in a white, one ounce tube with "Bacitraycin Plus" printed on the front in green. Lidocaine is one of the two active ingredients listed on the back of the tube. The tube measures approximately 5 inches long by 1 inch wide. The lot number is printed on the end of the tube. The following lot numbers are included in the recall: Lot Numbers 16001 through 16002 404001 through 404002 405001 through 405003 406001 through 406004 407001 through 407002 415001 through 415010 416001 through 416003 417001 through 417004 [Learn More]
About 500,000 units were affected by this recall.
First Aid Research Recalls Maximum Strength Bacitraycin Plus Ointment With Lidocaine Due to Failure to Meet Child Resistant Closure Requirement
Recalled Maximum Strength Bacitraycin Plus with Lidocaine. Lot number location on recalled Maximum Strength Bacitraycin Plus with Lidocaine
Maximum Strength Bacitraycin Plus Ointment with Lidocaine Recall Information
| Country of Origin | South Korea |
| Recall Date | 03/07/2018 |
| Recall ID | 8274 |
| Recall Number | 18118 |
| Hazard | The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain relieving ointment contains lidocaine, posing a risk of poisoning to young children if they put it on their skin or ingest it. |
| Injuries | None reported |
| Remedy | Consumers should immediately place the recalled ointment out of the reach of children and contact United Exchange, the product's importer, for instructions on how to receive a full refund from the place of purchase. |
| Remedy Option | Refund |
| Contact | United Exchange, the product’s importer, toll-free at 888-645-8204 from 8 a.m. to 5 p.m. PT Monday through Friday or online at www.firstaidresearch.com and click on “Recall” for more information. |
| Last Updated | 03/07/2018 |
| Similar To |
