VIQUA Solenoid Valve Kits
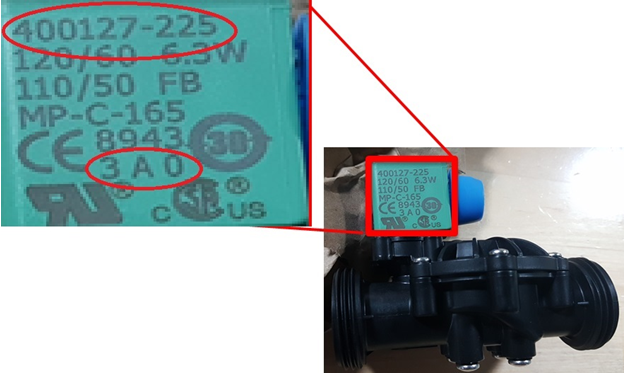
Description: This recall involves solenoid valves included in VIQUA solenoid valve accessory kits for residential and commercial UV water treatment systems. They can be identified by the green coil component attached to the solenoid valve with ASCO parts numbers 400127-xxx and date codes between 1A3 through 5A2 (13th week through the 52nd week of 2018) or 0B1 through 0B8 (1st week through the 8th week of 2019). [Learn More]
About 70 (in addition, about 170 were sold in Canada) units were affected by this recall.
VIQUA Recalls Solenoid Valve Kits for UV Water Treatment Systems Due to Electrical Shock Hazard
Recalled valve kit with product number displayed at the top of the green coil component and date code printed at the bottom.
VIQUA Solenoid Valve Kits Recall Information
| Country of Origin | United States |
| Recall Date | 10/03/2019 |
| Recall ID | 8676 |
| Recall Number | 20004 |
| Hazard | Electrical current could leak from the solenoid valve, posing an electrical shock hazard to the user. |
| Injuries | None reported |
| Remedy | Consumers should immediately stop using the recalled valve kits, disconnect power to the UV water treatment system and visit VIQUA's website for help inspecting the green coil component attached to the solenoid valve for the production date codes included in the recall. Consumers with the recalled solenoid valve should contact the firm to receive free installation of a replacement coil. |
| Remedy Option | Repair |
| Contact | VIQUA at 800-265-7246 from 8 a.m. to 4:30 p.m. ET Monday through Friday, email at [email protected] or online at https://viqua.com and click on “Product Safety” or http://info.viqua.com/safety-announcement for more information. |
| Last Updated | 10/03/2019 |
| Similar To |
