NumbSkin pain relief cream with lidocaine
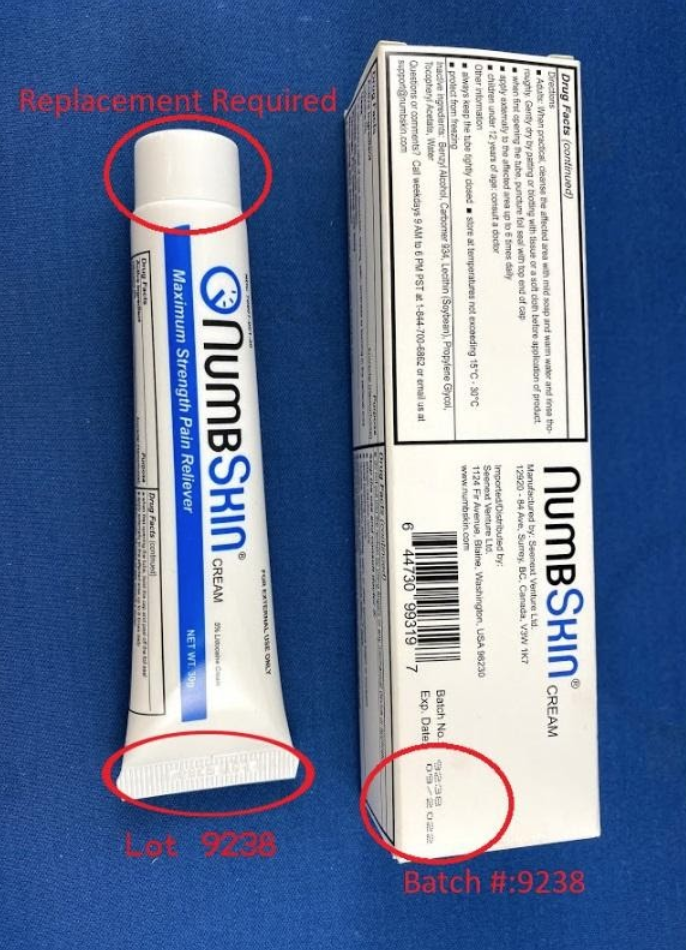
Description: This recall involves NumbSkin pain relief cream with 5% lidocaine. The topical anesthetic cream was sold in 30 grams in a white with blue tube. NumbSkin is printed on the tube. Lot 9238 and a date code of 10/2019 through 09/2020 in a MM/YYYY format is embossed on the tub's thin end. Batch number 9238 is printed on the product packaging. [Learn More]
About 8,000 units were affected by this recall.
NumbSkin Pain Relief Cream Recalled by SeeNext Venture Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning (Recall Alert)
Recalled NumbSkin pain relief cream.
NumbSkin pain relief cream with lidocaine Recall Information
| Country of Origin | Canada |
| Recall Date | 03/18/2020 |
| Recall ID | 8772 |
| Recall Number | 20724 |
| Hazard | The packaging is not child resistant as required by the Poison Prevention Packaging Act. The pain relieving cream contains lidocaine, posing a risk of poisoning to young children if they put it on their skin or ingest it. |
| Injuries | None reported. |
| Remedy | Consumers should immediately store the pain relief cream in a safe location out of reach of children and contact SeeNext Venture for instructions on how to dispose or return it and to receive a free replacement similar product with a child-resistant cap. Amazon is contacting all known purchasers directly. |
| Remedy Option | Replace |
| Contact | SeeNext Venture toll-free at 844-700-6862 from 9 a.m. to 6 p.m. PT Monday through Saturday, email at [email protected] or online at www.numbskin.com and click on Recall at the home page for more information. |
| Last Updated | 03/18/2020 |
| Similar To |
