Sanvall Rapid Alivio Pain Relieving Roll-On
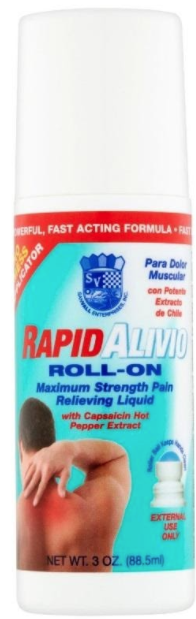
Description: This recall involves Sanvall Rapid Alivio Pain Relieving Roll-On in a 3 fl. oz (88.5 mL) white plastic bottle with a white cap and a red, white and blue label. "Rapid Alivio," "Maximum Strength Pain Relieving Liquid" and "Para Dolor Muscular" are printed on the label. Lot numbers 18032201, 18032301, 19040501, or 19052801 are printed on the bottom of the bottle. UPC code 605100014225 is printed on the side of the label. [Learn More]
5,400 units were affected by this recall.
Sanvall Enterprises Recalls Rapid Alivio Pain Relieving Roll-On Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning
Recalled Sanvall Rapid Alivio Pain Relieving Roll-On – 3 fl. oz (88.5 mL). Recalled Sanvall Rapid Alivio Pain Relieving Roll-On – label
Sanvall Rapid Alivio Pain Relieving Roll-On Recall Information
| Country of Origin | United States |
| Recall Date | 05/27/2020 |
| Recall ID | 8825 |
| Recall Number | 20126 |
| Hazard | The product contains the substance methyl salicylate which must be in child resistant packaging as required by the Poison Prevention Packaging Act (PPPA). The packaging of the product is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Injuries | None reported |
| Remedy | Consumers should immediately store the pain relieving roll-on in a safe location out of reach of children and contact Sanvall Enterprises for a full refund. |
| Remedy Option | Refund |
| Contact | Sanvall Enterprises collect at 305-887-1090 from 9 a.m. to 5 p.m. ET Monday through Friday, email at [email protected] and in the body of the email provide your name, address, and photo of the product or online at www.Sanvall.com and click on “Recall - Important Safety Information - Rapid Alivio Roll-On” for more information. |
| Last Updated | 05/27/2020 |
| Similar To |
