31 Medique Over-the-Counter drugs from the product lines Medi-First, Medi-First Plus, Medique, Dover, Otis Clapp, and Ecolab
Description: The recall involves 31 different over-the-counter drugs purchased on or after June 1, 2018 that are unexpired from the following brands: Medi-First, Medi-First Plus, Medique, Dover, Otis Clapp, and Ecolab. They include aspirin-containing products, acetaminophen-containing products, ibuprofen-containing products, lidocaine-containing products, diphenhydramine, loperamide, and naproxen products. The expiration date for tablets and creams can be found on either the top or side panels of the container carton in the format [YEAR/MO]. For products in spray bottles, the expiration date in the same format is located on the front of the bottle. The expiration date is found on the bottom for the spray cans. The 31 different recalled products are listed in the table below: Product Drug Package Type # of Packets Medi-First Non-Aspirin Acetaminophen acetaminophen (325 mg) 2 tablets packet 50 250 Medi-First Extra Strength Non-Aspirin Acetaminophen acetaminophen (500 mg) 2 tablets packet 50 125 250 Medi-First Sinus Pain & Pressure acetaminophen (500 mg) 2 tablets packet 50 125 250 Medique APAP acetaminophen (325 mg) 2 tablets packet 250 Medique Extra Strength APAP acetaminophen (500 mg) 2 tablets packet 50 125 250 Medique Back Pain-Off acetaminophen (250 mg) 2 tablets packet 50 100 250 Medique CCP Caffeine Fee acetaminophen (325 mg) 2 tablets packet 50 250 Medi-First Cold Relief acetaminophen (325 mg) 2 tablets packet 50 125 250 Medique Cramp Tabs acetaminophen (325 mg) 2 tablets packet 50 125 250 Medique Decorel Forte Plus acetaminophen (325 mg) 2 tablets packet 50 250 Medique Medicidin-D acetaminophen (325 mg) 2 tablets packet 50 100 250 Dover Aminofen acetaminophen (325 mg) 2 tablets packet 250 Otis Clapp Back Quell acetaminophen (200 mg) 2 tablets packet 150 Otis Clapp Mygrex acetaminophen (500 mg) 2 tablets packet 150 Otis Clapp Valihist acetaminophen (325 mg) 2 tablets packet 150 Medi-First Pain Relief Extra Strength acetaminophen (110 mg) aspirin (162 mg) 2 tablets packet 50 100 250 Medi-First Plus Pain Zappers acetaminophen (250 mg) aspirin (250 mg) 2 tablets packet 50 125 Medique Pain-Off acetaminophen (250 mg) aspirin (250 mg) 2 tablets packet 50 100 250 Medi-First Aspirin aspirin (325 mg) 2 tablets packet 50 125 250 Medi-First Plus Aspirin aspirin (325 mg) 2 tablets packet 50 125 Medique Aspirin aspirin (325 mg) 2 tablets packet 12 100 250 Medique Diphen diphenhydramine (25 mg) 1 tablet packet 24 200 Medi-First Ibuprofen ibuprofen (200 mg) 2 tablets packet 4 50 125 250 Medique I-Prin ibuprofen (200 mg) 2 tablets packet 3 100 250 Dover Addaprin ibuprofen (200 mg) 2 tablets packet 250 Medi-First Burn Cream with Lidocaine lidocaine (0.9 grams) packets 25 Medi-First Burn Spray lidocaine HCl (2%) 2 oz bottle -- Medi-First Blood Clotting Spray lidocaine (4%) 3 oz bottle -- Ecolab Burn Cream lidocaine (0.9 grams) packets 25 Medique Diamode loperamide HCl (2 mg) 1 tablet packet 6 50 100 Medique Mediproxen naproxen sodium (220 mg) 1 tablet packet 50 100 [Learn More]
About 143,300 units were affected by this recall.
Medique Recalls 31 Different Over-the-Counter Drugs Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning; Sold Exclusively on Amazon.com (Recall Alert)
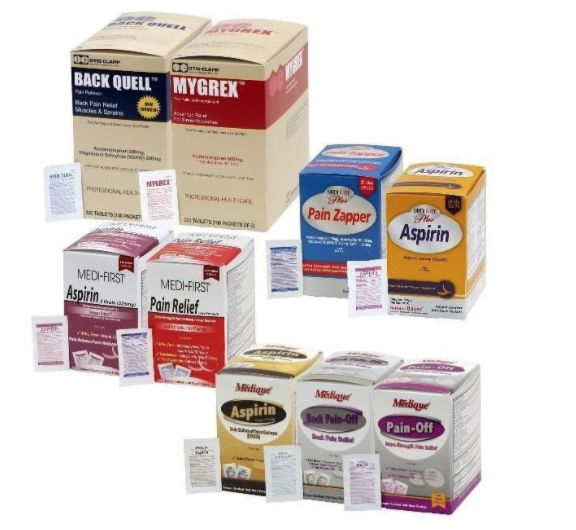
Recalled aspirin-containing products. Recalled acetaminophen-containing products
31 Medique Over-the-Counter drugs from the product lines Medi-First, Medi-First Plus, Medique, Dover, Otis Clapp, and Ecolab Recall Information
| Country of Origin | United States |
| Recall Date | 09/11/2020 |
| Recall ID | 8901 |
| Recall Number | 20780 |
| Hazard | The over-the-counter products contain regulated substances which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Injuries | None reported. |
| Remedy | Consumers should immediately store the recalled products in a safe location out of reach of children and contact Medique for information on how to dispose of the product and receive a full refund. All known purchasers are being notified directly. |
| Remedy Option | Refund |
| Contact | Medique at 800-680-2474 from 8 a.m. to 7 p.m. ET, Monday through Friday, or online at www.mediqueproducts.com and click on "Recall Notice" at the bottom of the page for more information including registration access. |
| Last Updated | 09/11/2020 |
| Similar To |
