Bella residential elevators
Description: This recall involves Bella residential elevators manufactured from 2009 to 2021. Models include Symmetry IGD, Hydraulic, and Winding Drum Elevators. The Symmetry brand name is located on the elevators' controllers. The elevators are used in consumers' homes. [Learn More]
About 10,500 units were affected by this recall.
Bella Elevator Recalls Residential Elevators Due to Child Entrapment Hazard; Risk of Serious Injury or Death to Young Children
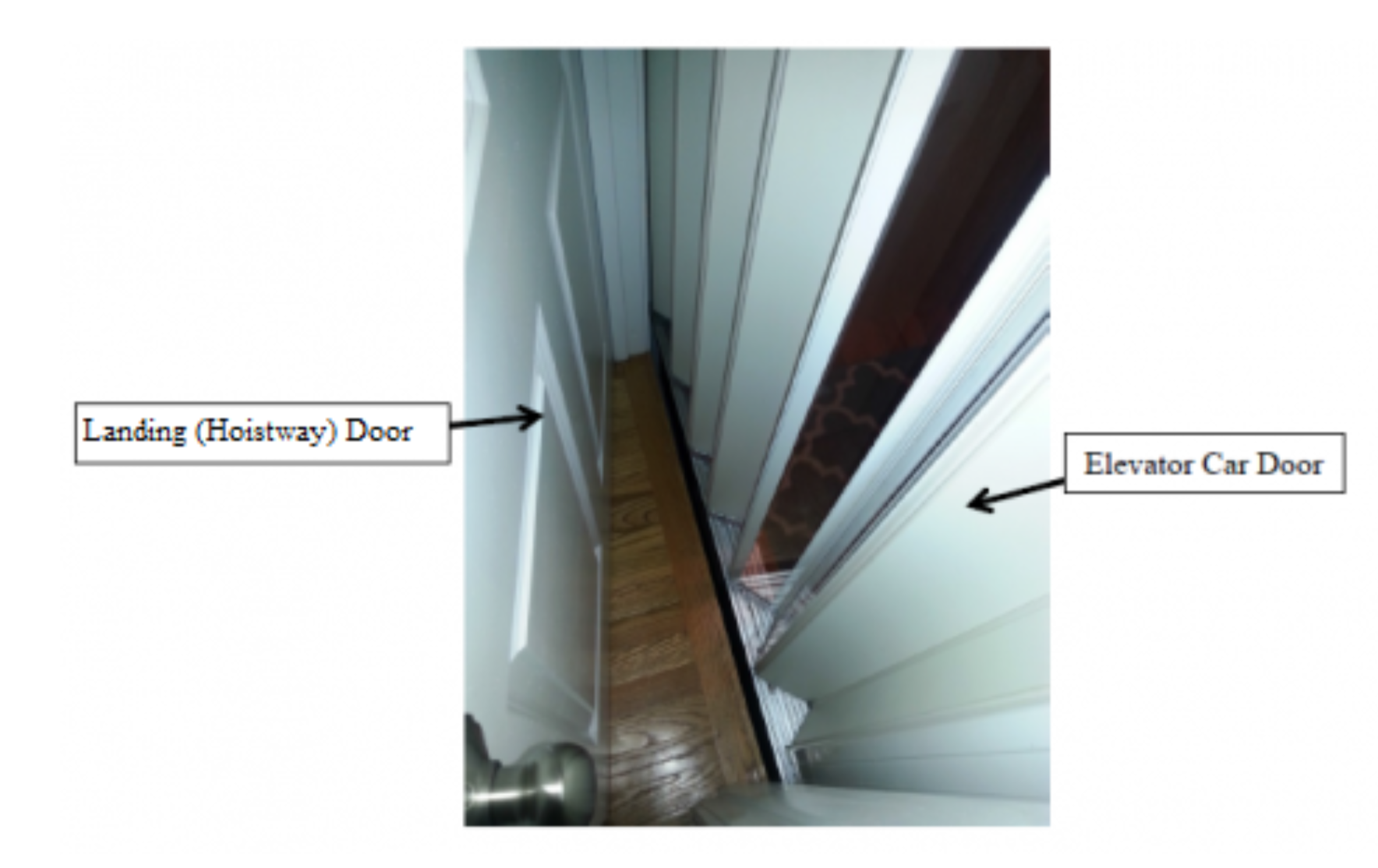
Residential Elevator with Space Between the Exterior Landing (Hoistway) Door and Interior Elevator Car (Accordion) Door. A young child can become entrapped if there is a hazardous gap between the doors.. Scenario depicting a child trapped between an exterior landing (hoistway) door and an interior elevator car door due to a hazardous gap. The exterior door locks the young child in the space between the doors when the elevator is called to another floor, putting the child at risk of being crushed or pinned and suffering serious injuries or death.
Bella residential elevators Recall Information
| Country of Origin | United States |
| Recall Date | 01/11/2022 |
| Recall ID | 9203 |
| Recall Number | 22045 |
| Hazard | Young children can become entrapped in the space between the exterior landing (hoistway) door and the interior elevator car door or gate if there is a hazardous gap, and suffer serious injuries or death when the elevator is called to another floor. |
| Injuries | None reported |
| Remedy | Consumers should keep unsupervised young children away from the recalled residential elevators and contact the manufacturers for instructions on how to measure for space guards to correct any hazardous gap. Space guards will be provided free of charge and assistance with space guard installation will be provided on request. |
| Remedy Option | Repair |
| Contact | Bella Elevator toll-free at 877-375-1428 from 8 a.m. to 5 p.m. CT Monday through Friday, or online at www.home-elevator-door-gap.com or www.symmetryelevators.com and click on "Recall" at the bottom of the page for more information. |
| Last Updated | 01/11/2022 |
| Similar To |
