Dritz Quick Cut™ Electric Scissors
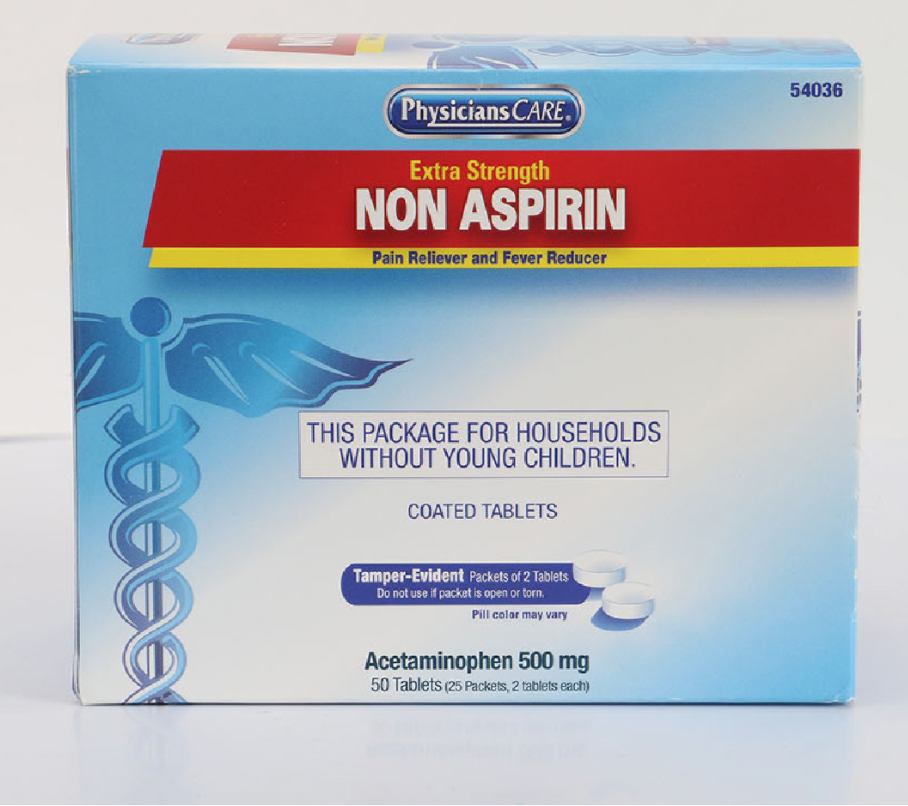
Description: This recall involves the PhysiciansCare brand Extra Strength Non Aspirin, Aspirin, Extra Strength Pain Reliever, Ibuprofen, Medication Station, and Multi-Pack over-the-counter drugs. The products contain aspirin, acetaminophen, or ibuprofen. They are packaged in cardboard boxes of 50, 100, 250, and 500 tablets per box. Product Drug Tablet Amount Extra Strength Non Aspirin Acetaminophen (500 mg) 50 tablets 100 tablets 250 tablets 500 tablets 2 boxes of 100 tablets each Aspirin Aspirin (325 mg) 50 tablets 100 tablets 250 tablets 500 tablets Extra Strength Pain Reliever Acetaminophen (250 mg) Aspirin (250 mg) 100 tablets 250 tablets Ibuprofen Ibuprofen (200 mg) 100 tablets 250 tablets 500 tablets 2 boxes of 100 tablets each Medication Station / Multi-Pack Acetaminophen (500 mg) Aspirin (325 mg) Ibuprofen (200 mg) Antacid (420 mg) 4 boxes of 100 tablets each with outer station 4 boxes of 100 tablets each without outer station The Antacid is not subject to this recall. [Learn More]
About 12,000 units were affected by this recall.
Acme United Corporation Recalls PhysiciansCare Brand Over-the-Counter Drugs Due to Failure to Meet Child Resistant Packaging Requirement; Risk of Poisoning (Recall Alert)
Recalled PhysiciansCare Extra Strength Non Aspirin in 50 Tablets (25 Packets, 2 tablets each). Recalled PhysiciansCare Aspirin in 250 Tablets (125 Packets, 2 tablets each)
Dritz Quick Cut™ Electric Scissors Recall Information
| Country of Origin | United States |
| Recall Date | 03/17/2022 |
| Recall ID | 9265 |
| Recall Number | 22733 |
| Hazard | The recalled over-the-counter products contain regulated substances (aspirin, acetaminophen, or ibuprofen) which must be in child resistant packaging when being used in the household as required by the Poison Prevention Packaging Act (PPPA). The packaging of the products is not child resistant, posing a risk of poisoning if the contents are swallowed by young children. |
| Injuries | None reported |
| Remedy | Consumers should immediately store the recalled products in a safe location out of reach of children and contact Acme United for information on how to dispose of or return the product and receive a full refund. Acme United is contacting all purchasers directly. |
| Remedy Option | Refund |
| Contact | Acme United toll-free at 888-520-2199 from 8 a.m. to 5 p.m. ET, Monday through Friday, or online at www.recallrtr.com/acmeunitedotc or at www.acmeunited.com and click on "Recalls" at the bottom of the page for more information. |
| Last Updated | 03/17/2022 |
| Similar To |
